Indium organometallics
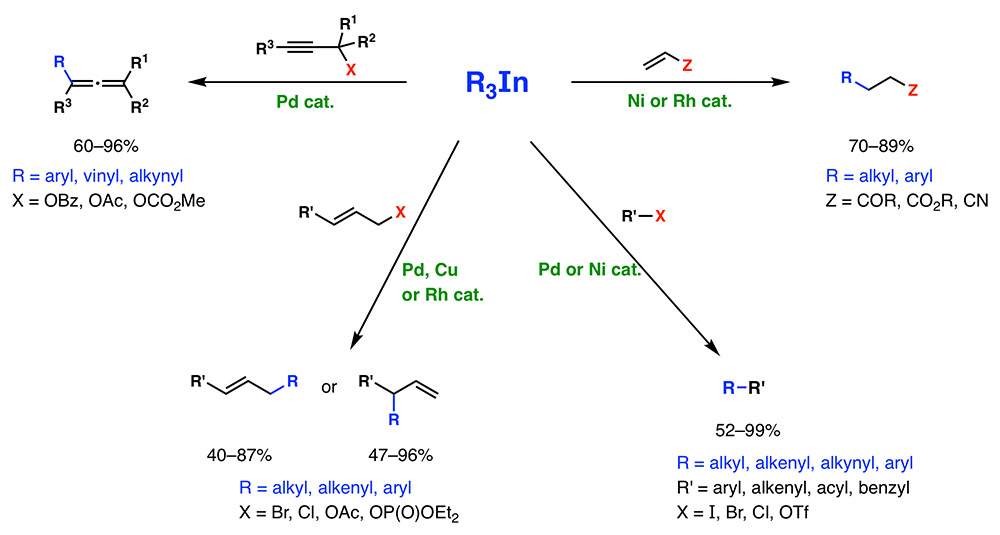
This research group has been a pioneer in the development of the reactions of organometallic compounds of indium with organic electrophiles catalyzed by transition metals. Triorganoindium reagents (R3In) can be efficiently prepared from organolithium or Grignard reagents by reaction with InCl3. Over the last 20 years, we have discovered that R3In participate in (i) conjugate addition reactions to α,β-unsaturated carbonyl compounds under Ni or Rh catalysis,[1] (ii) cross coupling reactions under Pd or Ni catalysis,[2] (iii) regioselective allylic substitution reactions under catalysis of Cu, Pd or Rh[3] and (iv) propargilic substitution reactions under catalysis of Pd, to obtain allenes.[4] In general, these reactions show a high versatility, being able to transfer to the electrophile carbon substituents of all types (sp3, sp2, Ar, sp) and a high atom economy, since, unlike other organometallics, R3In can transfer the three organic groups attached to the metal. Recently we have achieved important advances such as the preparation of solid, stable and R3In reactive in metal-catalyzed cross-coupling reactions[5] and transition-metal-free coupling reactions of R3In with chromene and isochroman acetals.[6]
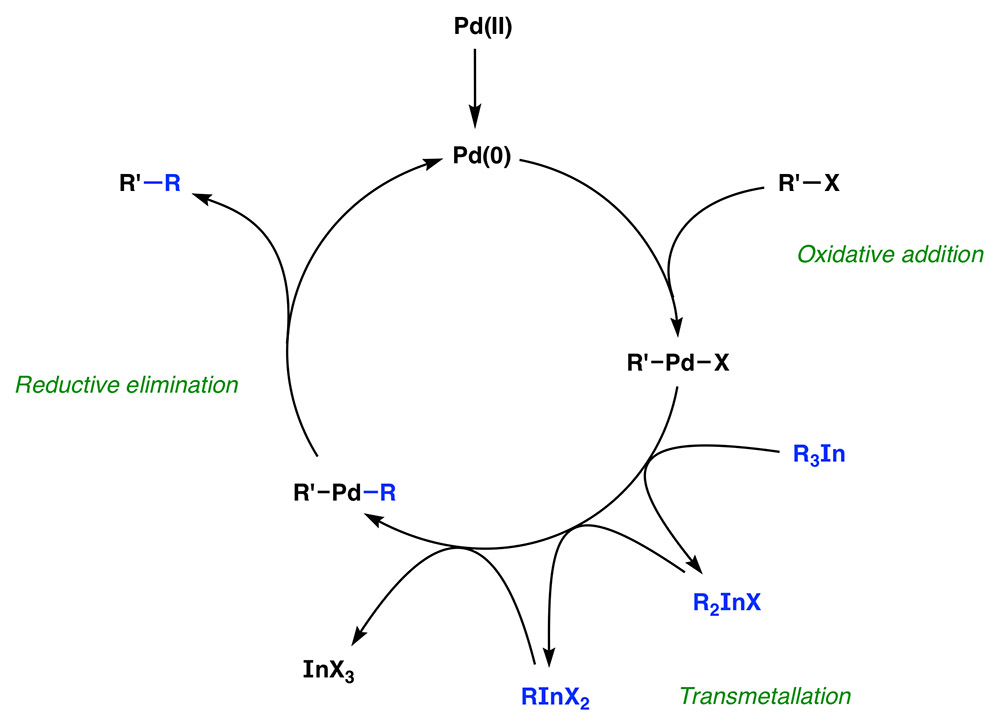
 The high atom efficiency behind this process can be explained with a mechanistic proposal that involves a catalytic cycle starting with an oxidative addition of the Pd(0) complex to the electrophilic partner, followed by transmetallation of an organic group from R3In to palladium and finally a reductive elimination step that releases the reaction product and regenerates the active catalyst (Scheme 9). We believe that the intermediate indium species R2InX and RInX2take part in the reaction until all the organic groups have been transferred. The driving force behind this process could be the significant difference in enthalpy that exists between indium organometallics and the InX3salts that are formed once the reaction is complete.
The high atom efficiency behind this process can be explained with a mechanistic proposal that involves a catalytic cycle starting with an oxidative addition of the Pd(0) complex to the electrophilic partner, followed by transmetallation of an organic group from R3In to palladium and finally a reductive elimination step that releases the reaction product and regenerates the active catalyst (Scheme 9). We believe that the intermediate indium species R2InX and RInX2take part in the reaction until all the organic groups have been transferred. The driving force behind this process could be the significant difference in enthalpy that exists between indium organometallics and the InX3salts that are formed once the reaction is complete.
 During these years, we have also demonstrated that R3In are useful reagents for the synthesis of bioactive products and new organic materials. For example, in 2014 the synthesis of neurodazine, a bioactive compound that is being analyzed for the treatment of Alzheimer’s, was carried out through a 4-step sequence in which four C–C bonds were formed by cross-coupling reaction using triorganoindium reagents.[7] Likewise, the cross coupling reaction with R3In has also allowed the synthesis of new organic materials such as dithienylethenes, molecular systems used as molecular switches.[8] Taken account the high selectivity of the coupling reactions using indium reagents, we have also prepared polythiophenes[9] and selectively substituted pyrimidines with interest in materials science.[10] Fundamental aspects in these synthetic procedures are the versatility and selectivity of the R3In in cross coupling reactions with haloalkenes.
During these years, we have also demonstrated that R3In are useful reagents for the synthesis of bioactive products and new organic materials. For example, in 2014 the synthesis of neurodazine, a bioactive compound that is being analyzed for the treatment of Alzheimer’s, was carried out through a 4-step sequence in which four C–C bonds were formed by cross-coupling reaction using triorganoindium reagents.[7] Likewise, the cross coupling reaction with R3In has also allowed the synthesis of new organic materials such as dithienylethenes, molecular systems used as molecular switches.[8] Taken account the high selectivity of the coupling reactions using indium reagents, we have also prepared polythiophenes[9] and selectively substituted pyrimidines with interest in materials science.[10] Fundamental aspects in these synthetic procedures are the versatility and selectivity of the R3In in cross coupling reactions with haloalkenes.

[1] (a) Ni(0)-cat.: Pérez, I.; Pérez Sestelo, J.; Maestro, M. A.; Mouriño, A.; Sarandeses, L. A. J. Org. Chem. 1998, 63, 10074. (b) Rh(I)-cat.: Tato, R.; Riveiros, R.; Pérez Sestelo, J.; Sarandeses, L. A. Tetrahedron 2012, 68, 1606.
[2] (a) Pérez, I.; Pérez Sestelo, J.; Sarandeses, L. A. Org. Lett. 1999, 1, 1267. (b) Pérez, I.; Pérez Sestelo, J.; Sarandeses, L. A. J. Am. Chem. Soc. 2001, 123, 4155. (c) Pena, M. A.; Pérez, I.; Pérez Sestelo, J.; Sarandeses, L. A. Chem. Commun. 2002, 2246. (d) Pena, M. A.; Pérez Sestelo, J.; Sarandeses, L. A. Synthesis 2003, 780. (e) Pena, M. A.; Pérez Sestelo, J.; Sarandeses, L. A. Synthesis 2005, 485. (f) Pena, M. A.; Pérez Sestelo, J.; Sarandeses, L. A. J. Org. Chem. 2007, 72, 1271. (g) Caeiro, J.; Pérez Sestelo, J.; Sarandeses, L. A. Chem. Eur. J. 2008, 14, 741. (h) Riveiros, R.; Saya, L.; Pérez Sestelo, J.; Sarandeses, L. A Eur. J. Org. Chem. 2008, 1959.
[3] Cu-cat.: (a) Rodríguez, D.; Pérez Sestelo, J.; Sarandeses, L. A. J. Org. Chem. 2003, 68, 2518. Pd-cat.: (b) Rodríguez, D.; Pérez Sestelo, J.; Sarandeses, L. A. J. Org. Chem. 2004, 69, 8136. Rh-cat: (c) Riveiros, R.; Tato, R.; Pérez Sestelo, J.; Sarandeses, L. A. Eur. J. Org. Chem. 2012, 3018.
[4] Riveiros, R.; Rodríguez, D.; Pérez Sestelo, J.; Sarandeses, L. A. Org. Lett. 2006, 8, 1403.
[5] Gil-Negrete, J. M.; Pérez Sestelo, J.; Sarandeses, L. A. Chem. Comm. 2018, 54, 1453.
[6] Gil-Negrete, J. M.; Pérez Sestelo, J.; Sarandeses, L. A.Org. Lett. 2016, 18, 4316–4319.
[7] Pérez-Caaveiro, C.; Pérez Sestelo, J.; Martínez, M.; Sarandeses, L. A.J. Org. Chem. 2014, 79, 9586.
[8] (a) Bouissane, L.; Pérez Sestelo, J.; Sarandeses, L. A. Org. Lett. 2009, 11, 1285. (b) Mosquera, A.; Fernández, I.; Canle, M.; Pérez Sestelo, J.; Sarandeses, L. A. Chem. Eur. J. 2014, 20, 15224.
[9] Martínez, M. M.; Peña-López, M.; Pérez Sestelo, J.; Sarandeses, L. A. Org. Biomol. Chem. 2012, 10, 3892
[10] (a) Mosquera, A.; Riveiros, R.; Pérez Sestelo, J.; Sarandeses, L. A. Org. Lett. 2008, 10, 3745. (b) Martínez, M. M.; Pérez-Caaveiro, C.; Peña-López, M.; Sarandeses, L. A.; Pérez Sestelo, J. Org. Biomol. Chem. 2012, 10, 9045. (c) Pérez-Caaveiro, C.; Moreno Oliva, M.; López Navarrete, J. T.; Pérez Sestelo, J.; Martínez, M. M.; Sarandeses, L. A. J. Org. Chem. 2019, in press (DOI: 10.1021/acs.joc.9b00643).



