Indium in Catalysis
Indium, as element in Group 13 presents oxidation states +1 and +3 with indium(III) being the most stable. Among its chemical properties, Indium (III) is a soft Lewis acid less reactive than Boron (III) or Aluminum (III) but exhibiting higher chemoselectivity, toleration organic functional groups such as alcohols or amines and can even be used in protic medium. Traditionally, indium(III) has been used as Lewis acid in a wide variety of reactions as σ-Lewis acid (σ-acid) in nucleophilic addition reactions to aldehydes and ketones, Diels-Alder and Friedel-Crafts reactions, etc. However, recent examples have shown that In(III) is also an efficient π-Lewis acid that coordinates with unsaturated systems such as alkynes, promoting the addition of different nucleophiles.

In the recent years this research group also focused the attention in the role of indium(III) as π-acid for the electrophilic activation of unsaturated systems.[1] The activation of unsaturated systems such as alkynes or alkenes towards nucleophilic addition is a research area of great interest. The electrophilic activation of unsaturated bonds allows the formation of carbon–carbon and carbon–heteroatom bonds by inter- and intramolecular fashion. In addition, these transformations can be integrated in sequential and tandem polycyclization reactions.

In 2015 we report the indium(III)-catalyzed intramolecular hydroarylation (IMHA) reaction of aryl propargyl ethers for the synthesis of 2H-chromenes (6-endo regioselectivity).[4] Interestingly, the catalytic activity of the indium(III) salts is function of the different counterions finding best results using indium triiodide. The mechanism for this intramolecular hydroarylation reaction suggests an electrophilic activation of the alkyne as first step followed by nucleophilic addition of the arene. Finally, rearomatization through a deprotonation step and protodematallation.

The indium-catalyzed intramolecular hydroarylation reaction with bromopropargyl aryl ethers and amines can be combined with palladium-catlyzed cross-coupling reactions with triorganoindium reagents providing a complete set of substituted chromenes and dihydroisoquinolines.[5]
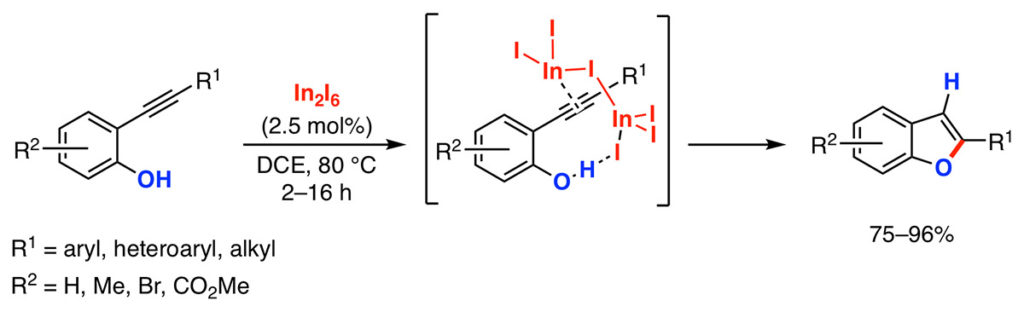
More recently, we also discovered that indium halides (InBr3, InI3) catalyze the hydroalkoxylation reaction of o-alkynyl phenols to give benzo[b]furans.[6] The reaction proceeds with excellent yields and computational studies indicate that the reaction proceeds through the coordination of the indium(III) to the alkyne preferentially on the hydroxyl group.
In addition, we also found that In(III) catalyzes the polycylization of aryl poliynes to give fused naphthochromenes in good yields and high regioselectivities (all endo).[7] Following this approach we found that the polyaromatic skeleton of chrysene can be obtained in good yield by the triple cyclization of a triyne.

[1] For a revision see: Indium(III) as π-acid catalyst for the electrophilic activation of carbon–carbon unsaturated systems. Pérez Sestelo, J.; Sarandeses, L. A.; Martínez, M. M.; Alonso-Marañón, L. Org. Biomol. Chem. 2018,16, 5733-5747.
[4] Alonso-Marañón, L.; Martínez, M. M.; Sarandeses, L. A.; Pérez Sestelo, J. Org. Biomol. Chem. 2015, 13, 379.
[5] Alonso-Marañón, L.; Sarandeses, L. A.; Martínez, M. M.; Pérez Sestelo, J. Org. Chem. Front. 2017, 4, 500.
[6] Alonso-Marañón, L.; Martínez, M. M.; Sarandeses, L. A.; Gómez-Bengoa, E.; Pérez Sestelo, J. J. Org. Chem. 2018, 83, 7970.
[7] Alonso-Marañón, L.; Sarandeses, L. A.; Martínez, M. M.; Pérez Sestelo, J. Org. Chem. Front. 2018, 5, 2308.