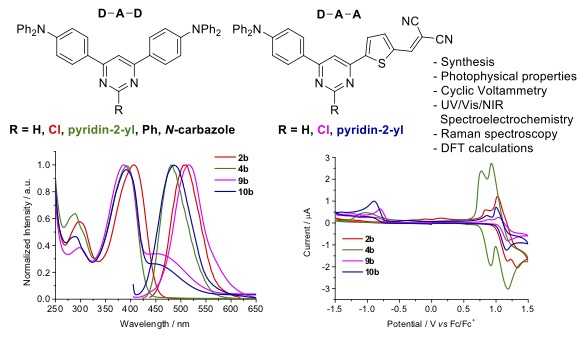
A series of donor-acceptor-acceptor (D-A-A) and donor-acceptor-donor (D-A-D) systems based on a pyrimidine -spacer with various substituents at C-2 position have been successfully prepared. The synthesis involved site-selective palladium cross-coupling reactions of chloropyrimidines with triorganoindium reagents and proceed in good yields and with atom economy. 4-(N,N-Diphenylamino)phenyl was chosen as donor group and thien-2-yl dicyanovinylene as acceptor one. The optical, vibrational, electrochemical and DFT calculations of these molecular systems were analyzed and experimental values show the important role of the substituents at C-2 position of the pyrimidine with stronger electron accepting ability, absorption in a wide range of UV-Vis, acceptable fluorescence lifetime and effective ICT properties. The ICT was observed in both series by the bathochromic shift on increasing the polarity of solvent. In addition, DFT calculations found a lower LUMOs of D-A-A molecules that suggest good electron ejection and transportation, being good properties for their application in various organic optoelectronic devices.