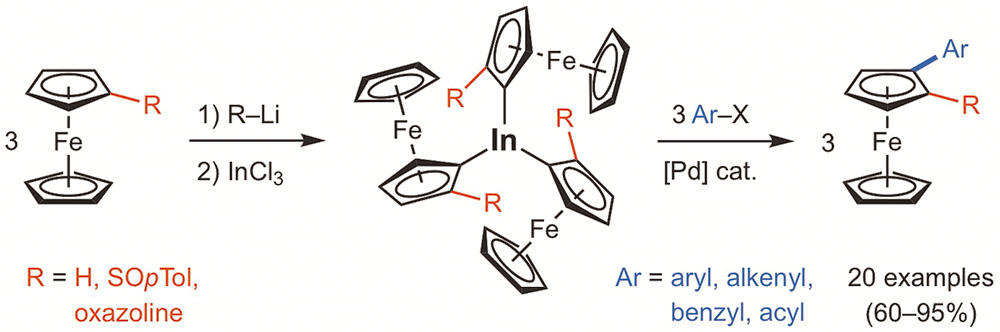
The preparation of ferrocenylindium species and palladium-catalyzed cross-coupling reactions for the synthesis of monosubstituted and planar chiral 1,2-disubstituted ferrocenes is described. Triferrocenylindium reagents (Fc3In) are efficiently prepared in a one-pot procedure from ferrocenes by lithiation and transmetallation to indium using InCl3 . The palladium-catalyzed cross-coupling reactions of Fc3In (40 mol%) with a variety of organic electrophiles (aryl, heteroaryl, benzyl, alkenyl and acyl halides) in THF at 80 °C overnight provided a wide variety of monosubstituted ferrocenes in good to excellent yields. This methodology allowed the stereoselective synthesis of planar chiral 2-aryl-1-oxazolylferrocenes and 2-aryl-1-sulfinylferrocenes, which are of interest in asymmetric catalysis.